
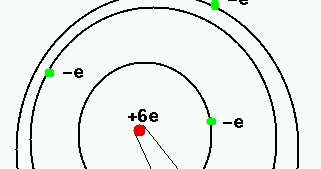
Both atoms have six protons in the nucleus, but have different. Carbon-12 is roughly 2 amu heavier than carbon-14. El único radionúclido cosmogénico que contribuye de manera significativa a la exposición interna de los seres humanos es el carbono 14. Carbon-14 has more neutrons than carbon-12. Carbon-14 decays at a faster rate than carbon-12. Now write the isotopic notation for carbon-14. Why are carbon-14 and carbon-12 considered to be isotopes Select all that apply. The name carbon-14 tells us that this isotope's mass number is #14#. Above is the atomic symbol for helium from the periodic table, with the atomic number, elemental symbol, and mass indicated. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.Įxample 1: What is the isotopic notation for the isotope carbon-14?įrom the periodic table, we see that the atomic number (number of protons) for the element carbon is #6#. The atomic mass number of Carbon is 12 amu, the proton number is 6, and it has no charge.
''614'C' We can determine the number of neutrons as 14-68 neutrons. Now write the isotopic notation for carbon-14. The name carbon-14 tells us that this isotope's mass number is 14. However, because different isotopes have different numbers of neutrons, they can differ in mass number, which is the sum of the protons and neutrons in the nucleus. From the periodic table, we see that the atomic number (number of protons) for the element carbon is 6. All atoms of the same element have the same number of protons, which is the atomic number of that element.
#Carbon 14 protons how to#
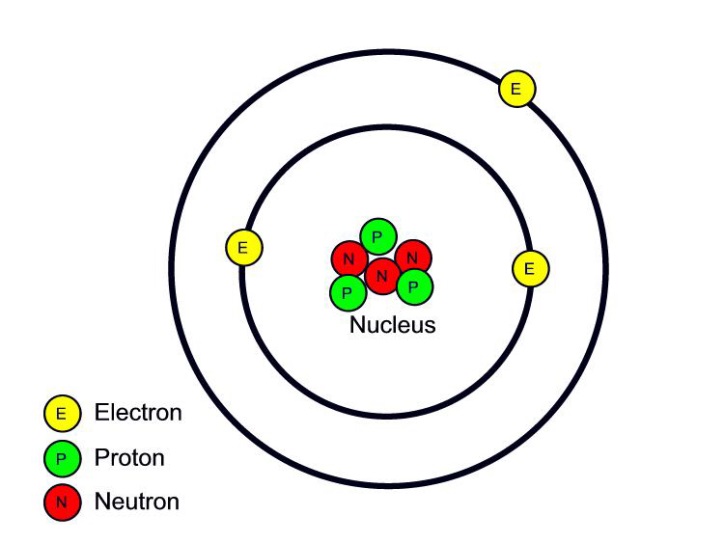
Isotopes are atoms of the same element that differ in the number of neutrons in their atomic nuclei. We call it carbon-14 because the total number of protons and neutrons in the nucleus, also known as the mass number, adds up to 14 (6+814). For each of the following isotopes, write the number of protons, neutrons, and electrons. Atoms are beautifully arranged on the periodic table: with our atom calculator, we will peek into them, learning how to find the atomic number, calculate the number of protons, neutrons, and electrons, and how to calculate the mass of an atom.


 0 kommentar(er)
0 kommentar(er)
